Eco-epidemiology of West Nile virus
West Nile virus (WNV) re-emerges annually in urban settings across the United States in a predictable cycle of accelerating mosquito-bird transmission known as amplification. When the virus amplifies sufficiently in birds and mosquitoes, it spills over into humans. Since 2005, I have been studying the ecology of WNV in urban environments across the U.S. These studies combine field collections of birds and mosquitoes with molecular studies of the virus and geographic analyses to elucidate the fundamental ecological drivers of WNV transmission, amplification, and evolution in urban settings.

Social-ecological factors of mosquito-borne virus transmission
My lab is building a research program in the Lower Rio Grande Valley (South Texas) addressing multiple elements of mosquito-borne virus transmission. We aim to understand the receptivity of diverse communities to emerging arboviruses such as Chikungunya and Zika viruses. We are also studying factors determining indoor and outdoor populations of vector mosquito abundance and developing new ways to monitor mosquito populations indoors. While collecting these mosquitoes in the valley, we are screening them for high profile viruses such as Dengue, Chikungunya, and Zika virus as well as viral symbionts such as insect-specific viruses.

Evaluation of mosquito control intervention campaigns
We are evaluating the success of several forms of mosquito control intervention. Our research team is conducting the randomized control trials for the Autocidal Gravid Ovitrap (AGO) in the Lower Rio Grande Valley as well as Autodissemination Stations using pyriproxyfen. We are also partnering with multiple local agencies to evaluate efficacy of additional intervention activities that are actively being used in Texas. We will be using mosquito outcome variables and possibly human outcome variables depending on arbovirus transmission activity during these studies.
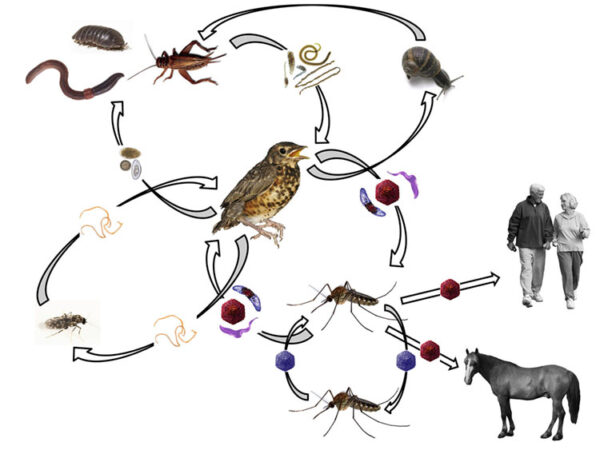
Pathogen interactions in arbovirus systems
My long-term program studying WNV has identified a suite of other pathgens that co-circulate in the same mosquitoes and birds responsible for amplication of WNV and spill-over to humans. We are transitioning these field studies to controlled laboratory co-infection experiments to identify the consequence of these co-infections on multiple parameters relevant to Vectorial Capacity of WNV.
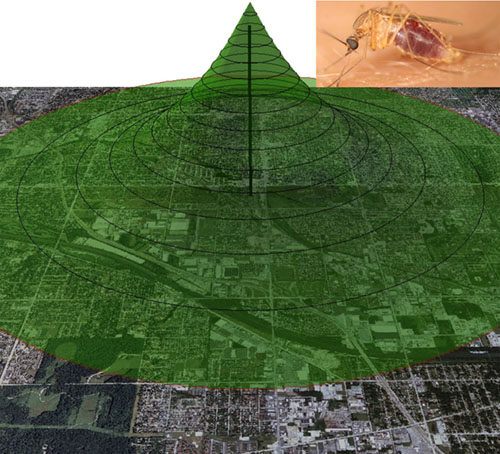
Mosquito movement and dispersal
Understanding mosquito dispersal and movement patterns is critical for effective mosquito control and vector-borne disease intervention. My lab has helped introduce stable isotope as a marking technique to study mosquito movement and dispersal. We have published this technique in the West Nile virus system and Culex pipiens mosquitoes in Chicago, IL and more recently the dispersal of Culex quinquefasciatus and Aedes albopictus in Texas. A new project just started studying Ae. aegypti dispersal and larval habitat sources in South Texas. I am also collaborating on several projects conducting stable isotope marking of Anopheles spp.mosquitoes in Africa. This stable isotope technique offers several advantages over existing marking technologies by being able to mark naturally occurring larval mosquitoes using a non-invasive approach and the marked adults can be uniquely identified for the life of the mosquito.
Vector-host interactions
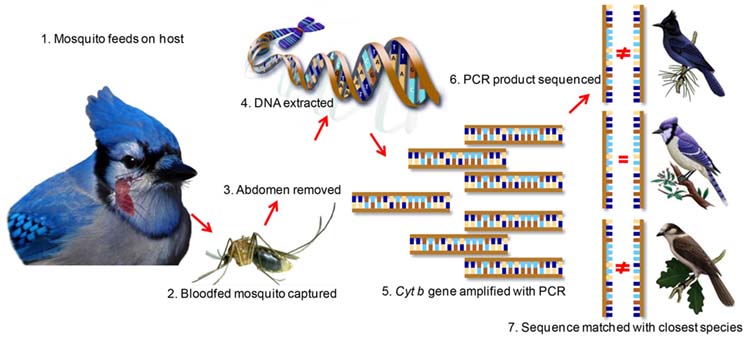
A prerequisite for effective disease prevention is to understand the ecology of natural cycles of pathogen transmission. One large component of the natural cycle is the identification of key vertebrate hosts that feed arthropod vectors, which provide blood meals and may also serve as pathogen reservoirs. Blood meal analysis is a tool used to identify the hosts of blood feeding arthropods. My lab uses a variety of approaches to improve the identification of the vertebrate species based on the blooded abdomen, which includes PCR-Sanger sequencing, quantitative PCR, stable isotopes, and Next Generation Amplicon Sequencing. Using these tools to inform the blood meal analysis, we build ecological models aiming to understand the contribution of different vector species and vertebrate reservoirs to the maintenance of vector-borne and zoonotic diseases.
Chagas disease eco-epidemiology
We are developing several projects aimed to understand the prevalence and mechanisms of maintenance of Trypanosoma cruzi, the agent of Chagas disease. Specifically, we will characterize kissing bug activity patterns, species distributions, host associations, and parasite infection prevalence and strain diversity. The TAMU College of Veterinary Medicine and Department of Entomology have a collaborative Citizen Science Program to learn about kissing bug and Chagas disease risk in the state. The team is interested in receiving safely-collected kissing bugs encountered by the public across the Southern U.S.

Conservation medicine
My lab is involved in multiple projects studying the pathogen and parasite communities in endangered species to improve our understanding of factors that regulate population growth. The primary project has focused on the Whooping Cranes (Grus americana) which is an endangered species that occurs only in North America and has rebounded from a low of 15 wild individuals in 1941 to a total of 245 in 2012. The Aransas-Wood Buffalo National Park population (AWBP) is the only wild self-sustaining population of whooping cranes, and these birds nest in and around Wood Buffalo National Park in Canada and winter in coastal marshes at Aransas National Wildlife Refuge in Texas. We are using multiple parasitological and diagnostic techniques to understand the baseline health of these birds with the goal of highlighting when and where these birds are exposed to agents of disease that could dampen their population recovery.